Success Stories
Re-engineered and Automated 21 CFR Part 11 Compliant Business Processes for a Leading CRO
The Client
The client, a global leader in clinical contract research and laboratory services, offers innovative R&D solutions and comprehensive central laboratory services for clinical studies. With a staff strength of about 4000, its operations and facilities are spread throughout North America, Europe and Asia.
TalenTek partnered with this Contract Research Organization to integrate its global operations through common processes and to construct a common system across all its sites. Business processes were re-engineered and a 21 CFR Part 11 compliant validated system was developed to:
- Automate business processes
- Improve operational efficiencies
- Achieve sustained growth
Challenges
The client had grown inorganically by acquiring laboratory units across different geographies. Therefore, the company did not operate as a single global entity; each business unit operated as a stand-alone site using its existing processes and systems. Multiple platforms and multiple independent applications were in use. Ensuring data integrity was cumbersome and difficult and resulted in data duplication, which in turn made reconciliation impossible. The organization lost business from pharmaceutical majors, which required CROs to seamlessly replicate trials at multiple geographies.
After several failed attempts to manage this issue internally, the client chose leverage TalenTek consultants for its proven strengths in executing end-to-end projects and strong project management expertise.
Our Solution
TalenTek consultants help solved the problem in two parts - by re-engineering a uniform business process to be used by all the units and then building an application that would support the new process. In the first phase, a dedicated team of business and domain consultants and technical architects worked with the client to capture the as-is process and pain points. The business requirements were then logically divided into five key process areas:
- Bid Management
- Study Setup
- Kit Management
- Sample Management
- Study Management
Working closely with subject matter experts (SMEs) in key process areas across all locations, TalenTek arrived at a new 'to-be' process that captured business needs and best practices from all locations. A global consensus was obtained by conducting road shows of the new process at key end-users' locations. Objections were noted and resolved. In parallel, the team carried out a performance engineering and capacity planning exercise to identify technical needs.
In addition to the complex business requirements of the clinical development domain, the application also needed to support non-functional requirements like Integrity, Security, Confidentiality and Non-Repudiation. TalenTek evaluated existing COTS (Commercial Off-The-Shelf) products for a build-versus-buy decision and together with the client, developed a custom solution based on Microsoft .NET platform. The solution needed to have role-based access control, with the ability to define roles and access rights without a code change. The system was also expected to be 21 CFR Part 11 compliant.
The new application, in addition to satisfying all business needs, had to be interfaced with the existing finance system for billing. It had to support Oracle Financials which was expected to replace the existing finance system. It needed to be interfaced with external LIMS systems and have the ability to read data transmitted by third party labs. In addition to all this, it needed to integrate with email, fax and print servers to initiate and track communication by these modes with investigators and study personnel.
TalenTek's consultants worked closely with the client SMEs to efficiently gather and detail all requirements. Industry standard tools were used to bring traceability, audit trail and efficiency into the process - Rational Requisite Pro was used for managing requirements, Rational Rose and XDE for design, Rational Test Manager for managing the test cases and SODA for generating hard copy documents. The use of V-model ensured that test planning was underway right from the requirements phase. Web versions of Rational tools and version control tools were used to allow the globally scattered team to work in parallel, on a single repository.
During design, the application was further split into sub-modules for capturing processes. The graphic here shows some of the key processes supported by the application.
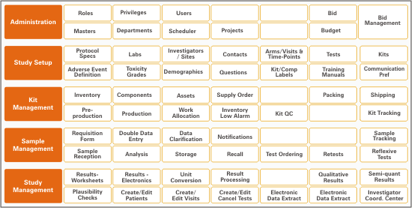
The final application supported all the client's requirements. The solution was designed to support six client locations across the globe and comprises over 700 internal users with around 60 concurrent users. Besides internal users, the application will be used by about 700 external users from various investigational sites across the world. The current technical back-end supports about 20 new studies every month and the generation and distribution of over 1000 lab reports daily.
Benefits
It is estimated that the Return on Investment for the application will be realized in about 3.5 years. This will be achieved by:
- Increased opportunity landscape - empowered by the application's ability to undertake complex trials and offer novel features
- mprovement in processes efficiencies by 15%-30%
- Bagging assignments for global studies from big pharmas that demand global process consistency, singular systems and regulatory compliance
- Reduced turn-around time through improved reliability and quality of service
- Rationalizing, leveraging and optimizing global infrastructure facilitated by common processes and systems
- Reducing license and technology replacement costs by retiring a multitude of legacy applications across different locations
- Reduced support costs required by the architecture supported on .NET
- Documented, Validated and Regulatory Compliant System & Processes, a key requirement in the Pharma industry, will ensure regulatory compliance and lowered risk for the customer. The client has already received positive feedback from certain pharma majors, which have audited the application
- Greater cash flow efficiency and customized periodic billing based on milestones
- Some of the immediate operational benefits to the client were:
- Enhanced workflow control
- Reduced data errors due to automated workflows
- Improved information exchange across organizational and geographical silos
- Better research and studies enabled by centralization
- Real-time access to information for all stakeholders
To hear more Contact TalenTek Today.
services
Technologies








